Our services are designed to meet your product’s unique requirements, whether you’re scaling up sterile injectable manufacturing, ensuring inspection compliance, or preparing for global distribution. Explore our full capabilities below.
Sterile Manufacturing
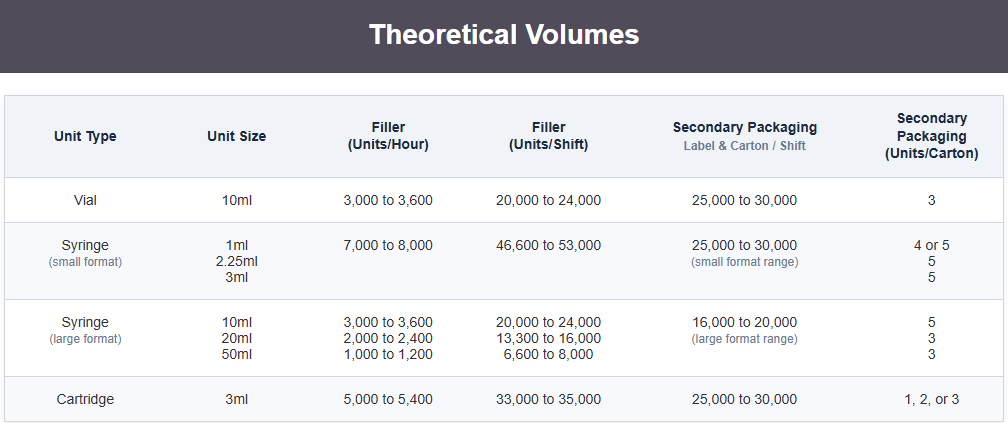
All aseptic filling takes place in an ISO 5 closed Restricted Access Barrier System (cRABS) with robotic automation. Our flexible fill/finish capabilities cover vials, syringes, and cartridges — delivering precision regardless of drug complexity.


.webp?width=2000&height=1333&name=packaging-VIAL-4%20(1).webp)
Visual Inspection Services
We provide both manual and automated visual inspection to guarantee sterility and the absence of visible particulates.
Capabilities
- Automated Vial Inspection: 2ml to 30ml vials; up to 75 ppm
- Automated Syringe/Cartridge Inspection: Syringes 1ml–10ml; cartridges 1.5ml–5ml; up to 75 ppm
Commercial Packaging Services



Packaging Capabilities